Phospholipid chemical structure
Time:2024-01-22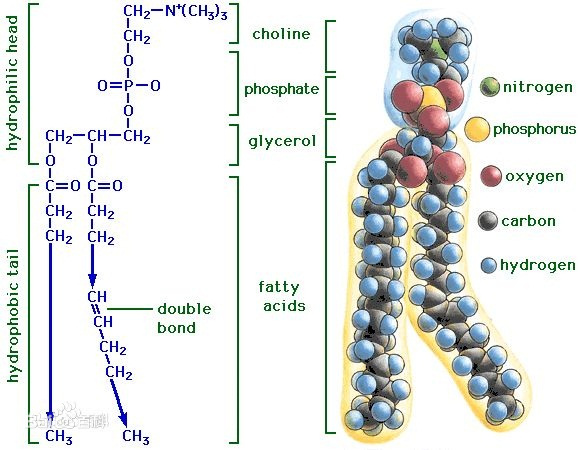
Phospholipids are a class of lipids that are a major component of cell membranes.They consist of a glycerol molecule, two fatty acid chains, a phosphate group, and a polar head group.The chemical structure of a phospholipid can be described as follows:
Glycerol Backbone: Phospholipids have a glycerol molecule as their backbone, which contains three carbon atoms.Each carbon is bonded to a hydroxyl (-OH) group.
Fatty Acid Chains: Two fatty acid chains are attached to the glycerol backbone.These fatty acids are hydrophobic (water-repelling) and consist of long hydrocarbon chains.The fatty acid chains can be saturated (with no double bonds between carbon atoms) or unsaturated (with one or more double bonds between carbon atoms).
Phosphate Group: A phosphate group is attached to the third carbon of the glycerol backbone. The phosphate group is hydrophilic (water-attracting) and is responsible for the polar nature of the phospholipid.
Polar Head Group: In addition to the phosphate group, there is a polar head group attached to the phosphate.The nature of the head group varies and determines the specific type of phospholipid.Common head groups include choline, serine, inositol, and ethanolamine.
The general structure of a phospholipid can be represented as:
-O- -O-
|| ||
CH2-O-PO3 2- -CH2-CH=CH-CH2-CH=CH-(Fatty Acid 1)
|| ||
CH2-O- -CH2-CH=CH-CH2-CH=CH-(Fatty Acid 2)
|| ||
(Head Group) (Head Group)
This structure results in a molecule with a hydrophilic "head" and hydrophobic "tail," making phospholipids amphipathic.In biological systems, phospholipids spontaneously arrange themselves into bilayers, forming the basis of cell membranes.


 CN
CN





